Equipment and services -Cellular and molecular imaging core facility
Equipment, services and software
Equipment, services and software
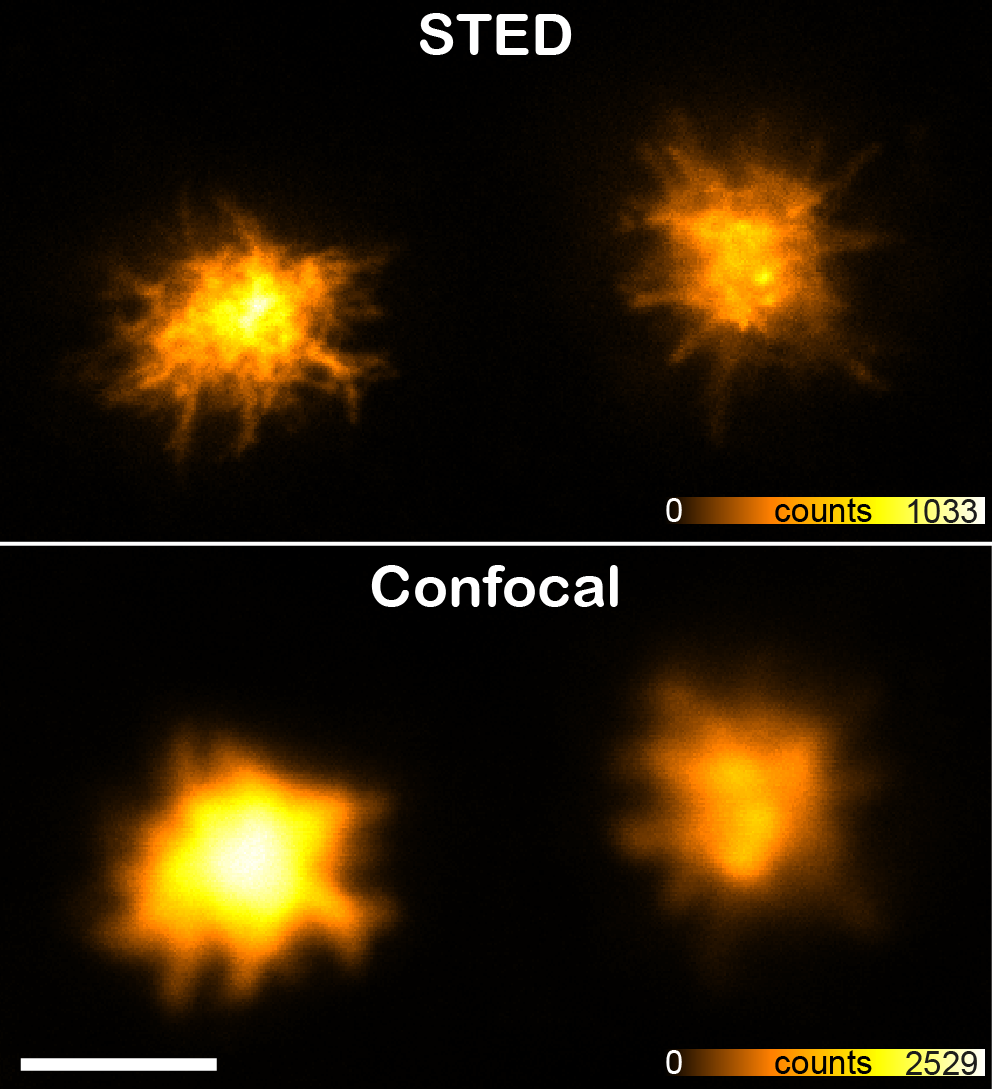
STED supreresolution nanoscopy
STED is the abbreviation of STimulated Emission Depletion and is a technique applied to microscopy, providing better resolution than traditional confocal microscopy. The STED resolution improvements come from a reduction of the physical dimensions in the focal spot from where the fluorescence can be emitted. This is achieved by introducing a depletion donut mask overlapping the diffraction-limited spot in the focus plane. By increasing the laser power of the depletion laser the donut gets bigger and consequently the spot gets smaller. This is achieved in all three dimensions.
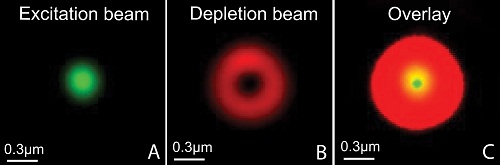
Our new Leica SP8 STED 3X microscope is equipped with a pulsed white-light laser that works in conjunction with an AOBS (acoustic optical beam splitter) providing any wavelengths between 470 and 670nm. The STED is provided by 3 powerful depletion lasers with wavelength 592, 660 and 775nm covering fluorophores of most of the visual spectra. It is fitted with sensitive hybrid detectors (HyDs) with time gating of the fluorescence signals and a 3D vortex for adjusting the ratio between the lateral and axial resolution. The microscope also has a high speed resonant scanner, a fast piezo z-drive and an incubator for live cell imaging.
STED superresolution microscope:
Location: Kunnskapssenter 3rd. floor, room 423.03.031 Confocal Imaging lab
Confocal laser scanning microscope equipped with a gated 3D STimulated Emission Depletion (STED) module for super resolution.
The programmable AOBS (acousto-optical beam splitter) provides up to 8 simultaneous excitation lines. The tuneable and pulsed white light laser (WLL) together with the spectral detection system enables freely tunable excitation from 470-670nm (1 nm steps) along with freely adjustable emission detection bands. Additional features include Lightgate functionality, which allows for temporal gating of the excitation light and Lambda Square that enables spectral mapping of excitation and emission information. The system has 4 internal detectors: 2 HyD (high sensitivity, hybrid GaAsP photon counting detectors) and 2 PMTs; high speed resonant scanner (8 KHz) and SuperZ galvo stage for fast lateral and axial acquisition; Adaptive Focus Control (AFC); a Ludin incubation chamber (temperature controlled, active CO2 gas regulator) as well as Huygens deconvolution software for 3D STED data sets.
Objectives: 10x|0.4 (air), 10x|0.4 (multi), 20x|0.75 (multi), 40x|1.3 (oil), 63x|1.4 (oil), 63x|1.3 (glyc), 100x|1.4 (oil).
Laser lines available: 405nm diode, WLL2 (white light laser) tunable from 470-670nm.
STED depletion lasers: 592nm, 660nm, 775nm.
Software
Software
CMIC uses Huygens Professional for deconvolution, image analysis, image processing and visualization.
All our users can get access to Huygens Professional on their own PCs (Windows, Linux or Mac) via the Huygens Everywhere login. Please contact Bjørnar Sporsheim for access and more info about this feature.
Features
- Deconvolution (GPU acceleration, workflow and batch processing, and more)
- 3D visualization (Volume renderer, surface renderer, and more)
- Image Analysis (Interactive object analyzer, colocalization analyzer, object tracker)
- Image processing (bleaching effects, spherical aberration, drift, crosstalk, uneven field illumination, and more)
The Huygens Professional is installed on a Dell Precision 5820, with following specs:
Dell Precision 5820 Tower XCTO Base Intel Xeon Processor W-2255 (10C 3.7GHz 4.7GHz Turbo HT 19.25MB 165W DDR4-2933) 256GB 4x64GB DDR4 2933MHz RDIMM ECC Memory 3.5" 12TB 7,200rpm SATA AG-Enterprise Hard Drive M.2 1TB PCIe NVMe Class 50 Solid State DriveM.2 2TB PCIe NVMe Class 40 Solid State DrivePNY GeForce RTX 2080 Ti Blower V2.
Access:
This image analysis machine is bookable via Booktilab (direct link).
For access to Huygens Everywhere please contact Bjørnar Sporsheim.
Location:
Øya - Gastrosenteret - Room 432.03.014.
TIRF microscopy
TIRF microscopy
Total Internal Reflection Fluorescence (TIRF) microscopy is the ideal tool to study cellular processes in the plasma membrane area. Because only a thin layer of the cell above the coverglass is illuminated, the rest of the cell is not disturbed. This fact results in a good signal to noise level, low bleaching, less stress to the cell and enables long time live imaging.
Our new Zeiss TIRF 3 is a laser based microscope that also works in epi-fluorescence mode. The system has 4 high powered lasers for rapid live cell photo bleaching experiments and two sensitive EMCCD cameras with a chromatic splitter for acquiring two fluorescent probes simultaneously. This microscope is equipped with excellent optics for enhanced resolution in TIRF mode and is also fitted with an incubator.
TIRF equipment
Location: Kunnskapsenter 3rd. floor, room 423.03.031 Confocal Imaging lab
Total internal reflection fluorescence (TIRF) microscope for (long-term) imaging of the plasma membrane region of live cells.
Motorized TIRF slider for reproducible setting of the TIRF angle. This microscope is also equipped with an incubator. Excellent optics for enhanced brightness.Dual cam module with 2x Hamamatsu EMCCD EMX2. DirectFRAP module. Hardware auto-focus Definitive Focus, and software auto-focus
Objectives: 10x|0.45 (air), 25x|0.8 (multi), 40x|1.4 (oil), 63x|1.3 (oil), 63x|1.46 (oil), 100x|1.57 (oil)
TIRF filters: GFP/YFP, CFP/GFP/DsRED, GFP/mRFP/Alexa633, DAPI/FITC/Rhod/CY5
Regular filters:
Laser lines available: 405nm (50mW), 488nm (100mW), 561nm (75mW), 638nm (75mW)
Confocal
Confocal
Location: Kunnskapssenter 3rd. floor, room 424.03.006A BSL lab
This inverted CLSM system is equipped with 3 internal detectors: 2 HyD (high sensitivity, hybrid GaAsP photon counting detectors) and 1 PMTs; high speed resonant scanner (8 KHz) and SuperZ galvo stage for fast lateral and axial acquisition; Adaptive Focus Control (AFC); and a prism-based spectral detection system that enables freely adjustable emission detection bands.
Beam splitters: 15/85, LIAchroic 405/488/552, LIAchroic 405/488/552/638
Objectives: 10x|0.4 (air), 20x|0.75 (multi), 40x|1.3 (oil), 63x|1.4 (oil).
Diode lasers: 405nm (50mW), 488nm (20mW), 552nm (20mW), 638nm (20mW).
Location: Kunnskapsenter 3rd. floor, room 423.03.031 Confocal Imaging lab
Confocal laser scanning microscope with spectra detector (Meta). Axiovert 200 fully motorized microscope stand, differential interference contrast (DIC), 2 filter based channels with PMT, Meta channel with 32 PMT-array detector.
Laser lines available: Ar 458, 488 and 514nm, HeNe 543nm and HeNe 633nm.
Location: Kunnskapsenter 3rd. floor, room 423.03.031 Confocal Imaging lab

Confocal laser scanning microscope with spectra detector (Meta) and high speed line scanner (Live). Axiovert 200M fully motorized microscope stand, differential interference contrast (DIC), 2 filter based channels with PMT, Meta channel with 32 PMT-array detector, pinhole in front of all detectors, fast objective piezo z-drive. Laser lines available: Diode 405nm, Ar 458, 477, 488 and 514nm, diode 561nm and diode 640nm. The Live unit can scan up to 120 confocal frames pr. sec with image quality 512 x 512 pixels in two different channels. Line scanning speed is up to 60.000 lines pr. sec. All scan combinations of line, X, Y, Z and time are possible. Available laser lines are diode 405 nm, diode 489 nm, DPSS 532nm and diode 635 nm. Data rates of up to 100 Mb pr. sec. are being processed with a real-time computer.
Location: Laboratory building 4th. floor, St.Olavs hospital
Confocal laser scanning microscope with spectra detector (Meta). Axiovert 200 fully motorized microscope stand, differential interference contrast (DIC), 2 filter based channels with PMT, Meta channel with 32 PMT-array detector.
Laser lines available: Diode 405nm, Ar 458, 488 and 514nm, HeNe 543nm and HeNe 633nm.
Location: Kunnskapsenter 3rd. floor, room 423.03.031 Confocal Imaging lab
Confocal laser scanning microscope with spectra detector (Meta). Axiovert 200 fully motorized microscope stand, differential interference contrast (DIC), 2 filter based channels with PMT, Meta channel with 32 PMT-array detector.
Laser lines available: Ar 458, 488 and 514nm, HeNe 543nm and HeNe 633nm.
Location: Acute-Heart Lung building 3rd. floor, St.Olavs hospital
Confocal laser scanning microscope. Axiovert 100 fully motorized microscope stand, differential interference contrast (DIC), 1 filter based channel with PMT.
Laser lines available: Ar 458, 488 and 514nm, HeNe 544nm.
Location: Acute-Heart Lung building, 3rd. floor St.Olavs hospital
The confocal spinning disk microscopy is a fast fluorescence imaging system for live cell study. It is build on an inverted microscope and based on a Nipkow disk with thousands of pinholes that are correlated with microlenses on a second disk. Detection is by a sensitive EMCCD camera able to acquire 512 x 512 pixel images up to 33 frames pr. second.
Fluorescence
Fluorescence
Location: Gastro building north, 3rd. floor, St.Olavs hospital
Scan^R offers fully automated high-throughput fluorescence, phase contrast and polarization image acquisition, including time-lapse (in environmental chamber) and automated analysis, so-called high-content screening. Examples of applications: cell transfection efficiency studies, intracellular transport and location studies, RNA interference, bacterial infection, cell cycle.
Location: Gastro building north 3rd. floor, St.Olavs hospital
Inverted fluorescence microscope Olympus IX71, Exfo 120 xenon-Hg excitation light coupled by optic fiber, fast remote controlled excitation filter wheel, mono-, trippel- and quatro- emission filters, polarization filter, phase contrast, objectives with magnification ranging from 4X to 60X, high sensitivity monochrome CCD camera XM10, color CCD camera XC30 and computer with Cell^P Image visualization software.
Location: Kunnskapsenter 3rd. floor, room 423.03.005 Confocal Imaging LAB CMIC.
Auto Imaging System for long-term monitoring of cell cultures and time-lapse cell imaging.
The microscope is fully-automated, digital, inverted multi-channel fluorescence and transmitted light imaging system with color and B/W cameras. It can do high-resolution mosaic tiling, multi position well scanning, and possibility for cell counting with threshold and multi spot time-lapse studies. The microscope has inserts for slides, petri dishes, 6 – 96 well plates, 25 and 75ml flasks.
8 LED Light cubes with filters for [Ex/Em in nm]: 390/447 (TagBFP), 445/510 (ECFP), 470/510 (EGFP), 500/524 (YFP), 531/593 (RFP), 585/624 (Texas Red), 628/692 (Cy5), 710/775 (Cy7)
Objectives:4x|0.13 (air), 10x|0.25 (air), 20x|0.45 (air), 40x|0.65 (air), 60x|0.75 (air) all with long working distance, no immersion
Bilder test
Contact and Service Request
Contact and Service Request
Manager
Bjørnar Sporsheim
Phone: +47 93033045
Email: bjornar.sporsheim@ntnu.no
Visiting address
Kunnskapssenteret, 3rd Floor (3. etg.)
St. Olavs Hospital
Olav Kyrres gate 10
7030 Trondheim
Norway
Visiting address CMIC Histology lab
Laboratoriesenteret, 4th Floor East (4. etg)
St. Olavs Hospital
Erling Skjalgsons gate 1
7030 Trondheim
Norway
Mailing address
NTNU
Department of Clinical and Molecular Medicine,
Cellular & Molecular Imaging Core Facility
Post box 8905
7491 Trondheim
Norway
