Molecular mechanisms of inflammation in cancer progression and bone loss - CEMIR
Research theme 5
Molecular Mechanisms of Inflammation in Cancer Progression and Bone Loss
Cancer cells modulate the tumour microenvironment by secreting factors that promote chronic inflammation, inhibit immune surveillance and disrupt bone homeostasis.
Autophagy may support tumour progression by intrinsic cell mechanisms, and by cross talks between tumour cells and stroma. The aim of theme 5 is to understand interactions between tumour cells and the microenvironment at a molecular level.
Our ambitions
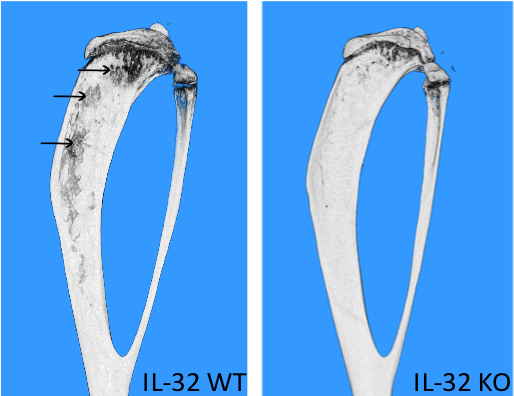
- To continue our studies on the role of IL-32 in multiple myeloma disease progression and mechanisms for IL-32 induction and secretion.
- Determine the role of immunoglobulins for bone loss in multiple myeloma.
- Describe the mechanism and clinical potential of IAP-antagonists on pathological bone degradation
- Determine how proinflammatory macrophage subtypes differs in their wiring towards different cell-death outcomes: anti-inflammatory apoptosis, proinflammatory necroptosis and inflammasome activation.
- Finish our studies of the role of the oxidative stress response system in aggressive breast cancer development.
- Disrupt hyper-activated genes in metastatic breast cancer cells encoding secreted immune regulators (including CSF1). Analyze the effect on tumour heterogeneity, tumour growth and metastasis.
- Characterize novel CSF1R chemical inhibitors generated at NTNU on primary macrophages in culture and evaluate in vivo effects.
- Determine how IL-6 trans-signaling induce autophagy in muscle cells in vitro and in vivo.
- Find how intracellular protein aggregates is released from myeloma cells and investigate the possible pathophysiological role and if other cell types can do the same.