Homopropargyl acetal cyclization - fluorination - Gold Catalysis in Organic Synthesis
Homopropargyl acetal cyclization - fluorination
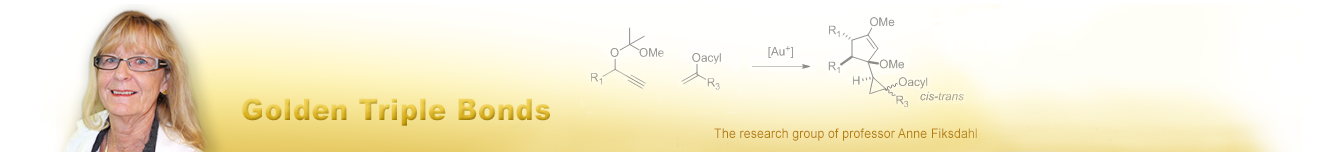
Based on our experience with propargyl acetals, we carried out gold(I) catalysed cyclization studies on homopropargyl acetals:

- A new gold(I)-catalysed regioselective transformation, based on a Petasis-Ferrier rearrangement / fluorination was developed to give3-fluoro-tetrahydropyran-4-one derivatives.
- The new one-pot protocol represents a useful method to readily afford highly substituted 3-fluoro-tetrahydropyran-4-ones (protected precursors of alkylfluoro-α,β-(E)-unsaturated ketones)
- as well as the analogues bromo- and iodo-derivatives from homopropargyl acetal
Such fluorinated products may be interesting as potential bioactive compounds or drug precursors, since fluoroalkyl (E)-α,β-unsaturated ketones are important structures, present in many bioactive agents.
Similar bromo- or iodo-compounds are suitable substrates in a variety of reaction in further synthesis.
Aaseng, J. E.; Iqbal, N.; Tungen, J. E.; Sperger, C.; Fiksdahl, A.
J. Fluorine Chem.; submitted.
"3-Fluorotetrahydropyran-4-one derivatives from
homopropargyl acetal"