Gold(I) catalysed cycloaddition reactions of propargyl substrates - Gold Catalysis in Organic Synthesis
Gold(I) catalysed cycloaddition reactions of propargyl substrates
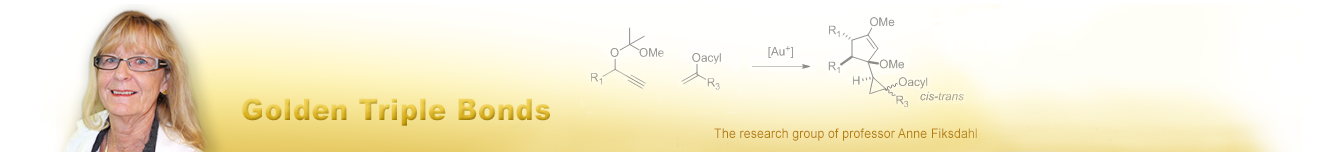
We are currently exploring the cycloaddition chemistry of propargyl substrates.
We have studied how the gold(I) catalysed reaction pathways are switching through
- cyclopropanation
- [2+3] cycloaddition
- [2+5] cycloaddition
- tandem cyclization
by varying the substrate combinations, as summarised in Sections 1-4 below:
The cycloadditions were proposed to take place by the following mechanistic pathways via the respective gold(I) carbenoid complex intermediates, formed by gold(I) catalysed propargyl acyloxy or alkoxy migration.
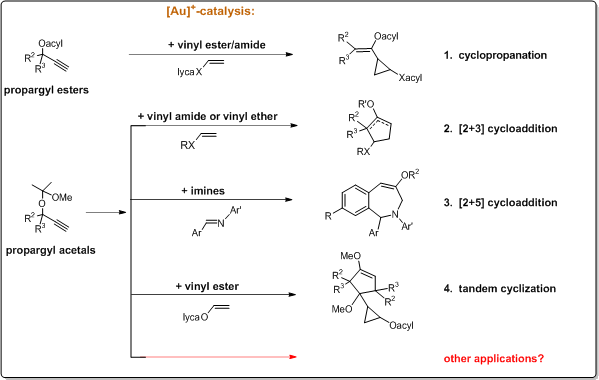
1. Gold(I)-catalysed cyclopropanation of propargyl esters
The cyclopropane unit is found in many natural occurring products with biological interesting properties and are also versatile substrates for a number of useful chemical transformations.
Alkene cyclopropanation of propargyl esters have been successfully perfomed with gold(I) catalysis. Our aim was to trap different propargyl esters with olefins directly connected to a heteroatom, such as vinyl acetates and vinyl sulphonamides.
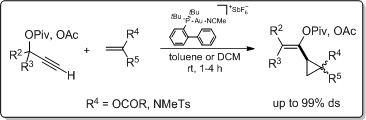
We demonstrated that gold(I) catalysed cycloadditions with propargyl esters and vinyl derivatives, directly connected to a heteroatom,
- efficiently provided highly substituted vinylcyclopropane derivatives;
- the diastereoselectivity (up to 99%) was controlled by the sterical more demanding olefin substituent.
These products may further be used as versatile building blocks.
Sperger; Tungen; Fiksdahl Eur. J. Org. Chem. (2011) 3719.
"Gold-catalysed reactions of propargyl esters with
vinyl derivatives"
2. Gold(I)-catalysed [2+3] cycloaddition of propargyl acetals
Studies on gold(I) catalysed reactions of propargyl acetals were previously scarce, in contrast to the corresponding esters. In the present project, we investigated gold(I) catalysed cyclization reactions of propargyl acetals and esters, respectively, in order to identify the ability of the two substrate groups to follow two different chemoselective cyclization pathways with vinylic reactants, such as vinyl amides and vinyl ethers.
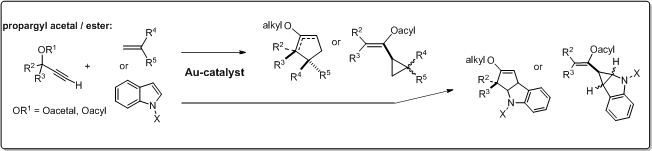
Through this comparative study on chemoselective gold(I) catalysed alkene cycloadditions of propargyl substrates, it was demonstrated that propargyl acetals follow
- a different new [2+3] cycloaddition pathways and
- show significantly higher reactivity than the corresponding esters.
N.Iqbal; C. Sperger; A. Fiksdahl; Eur. J. Org. Chem. (2013) 907.
"Gold-catalysed alkene cycloaddition reactions of propargyl acetals"
3. Gold(I)-catalysed [2+5] cycloaddition of propargyl acetals
As part of our investigations on the chemistry and the potential of the highly reactive propargyl acetals, we studied the transformation of phenylpropargyl acetals and benzaldimine substrates in the presence of a gold(I) catalyst.
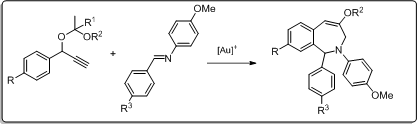
A new gold(I) catalysed formal intermolecular [2+5] cycloaddition reaction was developed.
- Benzo[c]azepin-4-ol products were readily prepared, and
- a direct one-pot procedure from the propargyl acetal and the respective aldehyde and aniline precursors was successful, as well.
- The ring closure takes place by an intramolecular Pictet-Spengler type reaction.
Our reported [2+5] cycloaddition represents a a new synthetic approach to access benzofused N-heterocyclic azepine derivatives by an entirely gold(I) catalysed reaction.
N. Iqbal; A. Fiksdahl J. Org. Chem. (2013) 78, 7885.
"Gold(I)-catalyzed benz[c]azepin-4-ol synthesis by intermolecular [5 + 2] cycloaddition"
4. Gold(I)-catalysed tandem cyclization of propargyl acetals
In order to expand the understanding of the chemistry of propargyl acetals and explore the possibilities of generating novel compounds through gold(I) catalysed reactions, we performed a study on chemoselective gold(I) catalysed cycloadditions of propargyl acetals with vinyl acetates, showing that such substrates afforded new tandem products:
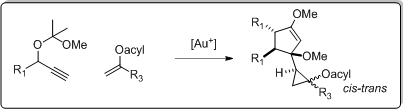
by following a new tandem cyclization pathway. A plausible mechanism including subsequent [1+2] and [2+3] cycloadditions, was proposed for these highly regio- and stereoselective gold(I) catalysed reactions.
The total outcome of the reactions were the
- formation of the cis/trans-cyclopropyl-cyclopentenyl tandem products
- by two subsequent cycloaddition reactions
- involving two units of propargyl acetals and one unit of vinyl esters.
The novel tandem reaction allows the construction of polysubstituted and highly functionalised bicyclic compounds.
M. Siah; M. Kaur; N. Iqbal; A. Fiksdahl Eur. J. Org. Chem. (2013) in print
"Gold(I)-catalysed tandem cyclization of propargyl acetals and vinyl esters"