Circulation physiology and resuscitation
Circulation physiology and resuscitation
Specific research programmes
Ultra-Power – Dynamic Regulation of the Cardiovascular System
Ultra-Power – Dynamic Regulation of the Cardiovascular System
The circulation in the cardiovascular system is driven by hydraulic energy supplied in bursts. The effectiveness of the energy production depends on the ventricular-arterial coupling, i.e. the interplay between the contractility and the vascular bed resistance to the blood flow. The total vascular resistance, the vascular impedance, consists of both static and dynamic elements; it depends both upon aortic elasticity and the elasticity of the blood vessels, peripheral resistance in the arterioles and capillaries, the pressure from reflected waves, blood viscosity, and the heart rate. Both vascular impedance and cardiac contractility can change considerably on account of sickness or medication. A disconnect in the interplay between the heart and the vascular bed can dramatically reduce the energy production and the cardiovascular effectiveness.
Today, clinical monitoring of cardiovascular function in critically ill patients is most commonly based on real-time readings of blood pressure and blood flow. Subsequently, only a fraction of all the information found in the continuous, pulsatile curves for blood pressure and blood flow is used. This results in the loss of important information on pulsatility and the peripheral regulation of the circulatory system, which in physiological animal studies has proven significant.
Our research group has in collaboration with the ultrasound group at NTNU developed software for real-time, continuous measurements on blood pressure and ultrasound-measured blood flow. The system, called Ultra-Power (uPWR – Ultrasound-based Cardiac Power), offers several possibilities:
-
Multiplication of the blood pressure curve with the curve for blood flow from the left ventricle produces an effect curve (Cardiac Power) which describes the amount of hydraulic energy transferred to the circulatory system per unit of time.
-
Calculation of the share of this energy that is connected to the pulsations in blood pressure and blood flow (Oscillatory Power) is an objective for dynamic ventricular-arterial coupling.
-
Impedance analyses, which indicate whether changes in impedance has a central or peripheral origin in the circulatory system.
This new tool makes exciting clinical research possible, allows development of new clinical monitoring modalities, and may establish new treatment objectives for critically ill patients. Thus far the Cardiac Power project has resulted in three PhD projects:
-
Audun Eskeland Rimehaug: “Beat-to-beat cardiac power – minimally invasive assessment of overall cardiovascular performance” (completed, dr. Rimehaug defended his dissertation on December 2, 2016).
-
Tomas Dybos Tannvik: “Ultrapower, from experimental physiology to patient monitoring. The exploration of a novel Doppler based assessment of human cardiovascular function” (completed, dr. Tannvik defended his dissertation on September 11, 2020).
-
Hans-Martin Flade: Ultrapower and oscillatory power fraction, new tools for optimizing cardiovascular function in the critically ill patient? (start: Jauary 2021)
There is room, for additional single studies and/or PhD projects.
Dynamic Autoregulation of Renal Blood Flow
Dynamic Autoregulation of Renal Blood Flow
Severe kidney failure is often not detected until it has run its course for a while. Animal studies have shown that the kidney failure is preceded by disruption in the renal blood flow autoregulation, which can be detected by simultaneously recording blood pressure and blood flow curves from the renal artery. In these studies, the blood flow has been measured with flow probes placed directly on the renal artery.
To make the method clinically usable, we will conduct non-invasive measurements of the blood flow in renal arteries by use of ultrasound. We do, however, require continuous measurements of flow in the renal artery over the course of several minutes, which is difficult because the subject’s breathing causes the kidneys to move. Therefore, we have sought collaboration with SINTEF’s Medical Technology department, which has developed expertise in imaging organs that move with breathing. St Olavs hospital, NTNU and Medical Imaging and SINTEF have established a joint project called Ultrasound based determination of dynamic autoregulation of kidney blood flow in man (ultra-DARK). The project is funded by grants from the health authorities of Mid-Norway (Helse Midt-Norge Innovasjon and Samarbeidsorganet – Helse Midt-Norfe RHF).
Gerald Dibona, the former head of the American Physiological Society (APS), and Professor Emeritus at the University of Iowa, has performed several of the animal studies that have laid the groundwork for using dynamic autoregulation of renal artery flow for earlier detection of severe kidney failure. He, and Professor Sven Erik Ricksten, Gothenburg, contacted us with a proposal of conducting human studies after learning of our development of equipment for simultaneous measurements of ultrasound-based blood flow and blood pressure. The project was conceived in collaboration with them, and they participate in the projects Scientific advisory Board. Robert Fridthiof, Researcher at Uppsala University Hospital, who has conducted experimental studies on dynamic autoregulation of renal artery flow in sepsis, is also attached to the project.
Analysis of Course of Events in Connection with Cardiac Arrest and Resuscitation
Analysis of Course of Events in Connection with Cardiac Arrest and Resuscitation
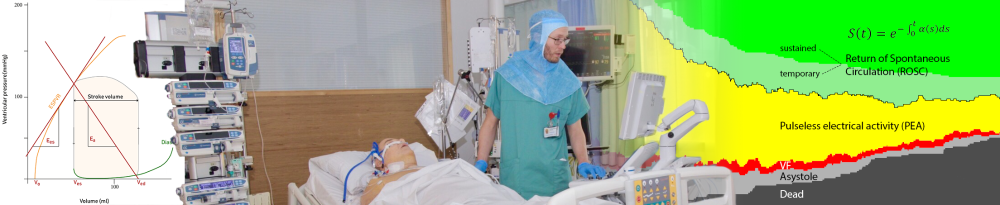
Every year resuscitation is attempted on approximately 2,500 persons outside hospitals in Norway, and (likely) on approximately 1,500 patients admitted to Norwegian hospitals. When the heart stops completely, it usually occurs either as a result of heart disease (heart attack, for example), or as a result of external factors (drowning, for example). Time is of the essence, and immediate action in the first few minutes (CPR, defibrillation, medication) is of utmost importance for the prognosis. Our research group has – and in close collaboration with medical and technical researchers in Norway and abroad – examined the dynamic development during CPR, the phase that determines whether the patient dies or reestablishes stable circulation.
With modern statistical methods for lifespan analysis, so-called multi-state models, we have described the course of events during CPR (for example the odds that the patient reestablishes stable circulation, or that he/she “loses” circulation after initial resuscitation). Furthermore, we are investigating the factors that influence this course of events (for example the cause of the cardiac arrest), and how the patient’s EKG changes over time.
The work has thus far resulted in several publications, and three PhD projects:
-
Trond Nordseth: “Clinical state transitions during the provision of advanced life support (ALS) to patients in cardiac arrest” (2014)
-
Daniel Bergum: “In-hospital cardiac arrest – causes, recognition and survival” (2016)
-
Gunnar Waage Skjeflo: “PEA development in cardiac arrest (In progress, expected 2019)
We have focused on the occurrence of pulseless electrical activity (PEA) as the first heart rhythm, in particular, since this occurs with many patients, and has not yet been adequately studied; and cardiac arrest in hospitals, since the quick arrival of emergency personnel provides the best opportunities to make observations on the dynamic course of events.
We will be conducting further study analyzing the immediate effect of the medication adrenaline on this course of events, as well as undertaking similar analysis of cardiac arrest and resuscitation in children.
Temperature deviation in trauma patients
Temperature deviation in trauma patients
Collaborations:
- Universitetet i Stavanger
- Universitetet i Oslo
- Children's Hospital of Philidelphia
- Uppsala Universitet
- Göteborgs Universitet
- Center for Resuscitation Science, University of Pennsylvania
- University of Iowa
- Bioengineering and Resuscitation, University of the Basque Country
Management
-
Idar Kirkeby-Garstad Associate Professor emeritus/senior consultant
+4791757579 +4772826821 idar.kirkeby-garstad@ntnu.no Department of Circulation and Medical Imaging -
Eirik Skogvoll Professor/ anesthesiologist
+4792404347 +4772574549 eirik.skogvoll@ntnu.no Department of Circulation and Medical Imaging

