Non-pharmacological treatment of sleep problems
Non-pharmacological treatment of sleep problems in primary care
Watch a short movie about insomnia in Norway, documenting the sleep course Sleep well and our research project:
Background for the study
Insomnia is the most common sleep disorder in the general population and in clinical practice. Cognitive behavioural therapy for insomnia (CBT-I) is the first-line treatment for long-term alleviation of chronic insomnia, but its availability to patients is limited. The Norwegian Health Directorate has therefore developed the sleep course Sleep well, a CBT-I-based treatment provided in primary care. The intervention is delivered in a group setting of up to 15 participants at a time. It is a low-threshold treatment, where adults with insomnia can sign up without a referral from their general practitioner.
In the research project ‘Non-pharmacological treatment of sleep problems in primary care’, we investigated the effectiveness of the group-delivered CBT-I among adults with insomnia in Norwegian primary care.
Brief description of methods
The study was conducted as a pragmatic, multicentre randomised controlled trial in the Norwegian primary care. 26 public, interdisciplinary primary care centres participated in the data collection (see map). All adults who signed up for the study were screened for the severity of their insomnia symptoms, using the Insomnia Severity Index (ISI). Those with an ISI score ≥ 12, residence in a municipality with a participating centre and adequate Norwegian language skills were considered for participation. Untreated sleep apnea, ongoing cancer treatment, current attack of multiple sclerosis, psychotic, bipolar and personality disorders, and dementia or other neurodegenerative conditions were applied as exclusion criteria.
There were six measurement timepoints in the study:
- Baseline measurements after randomisation and prior to intervention start
- Immediately post-intervention (4 weeks after baseline)
- 3 months post-intervention (primary outcome measurement)
- 6 months post-intervention
Effectiveness of group-delivered CBT-I on insomnia severity
Participants receiving group-delivered CBT-I (intervention group) were compared with those on a waiting list (control group) to investigate whether the intervention reduces insomnia severity. 308 participants were included in the trial. Of these were 127 randomised to the waiting list, and 181 to group-delivered CBT-I. In the whole sample, mean age was 49.5 (SD 13.2) years, and 75% of participants were female and 25% were male.
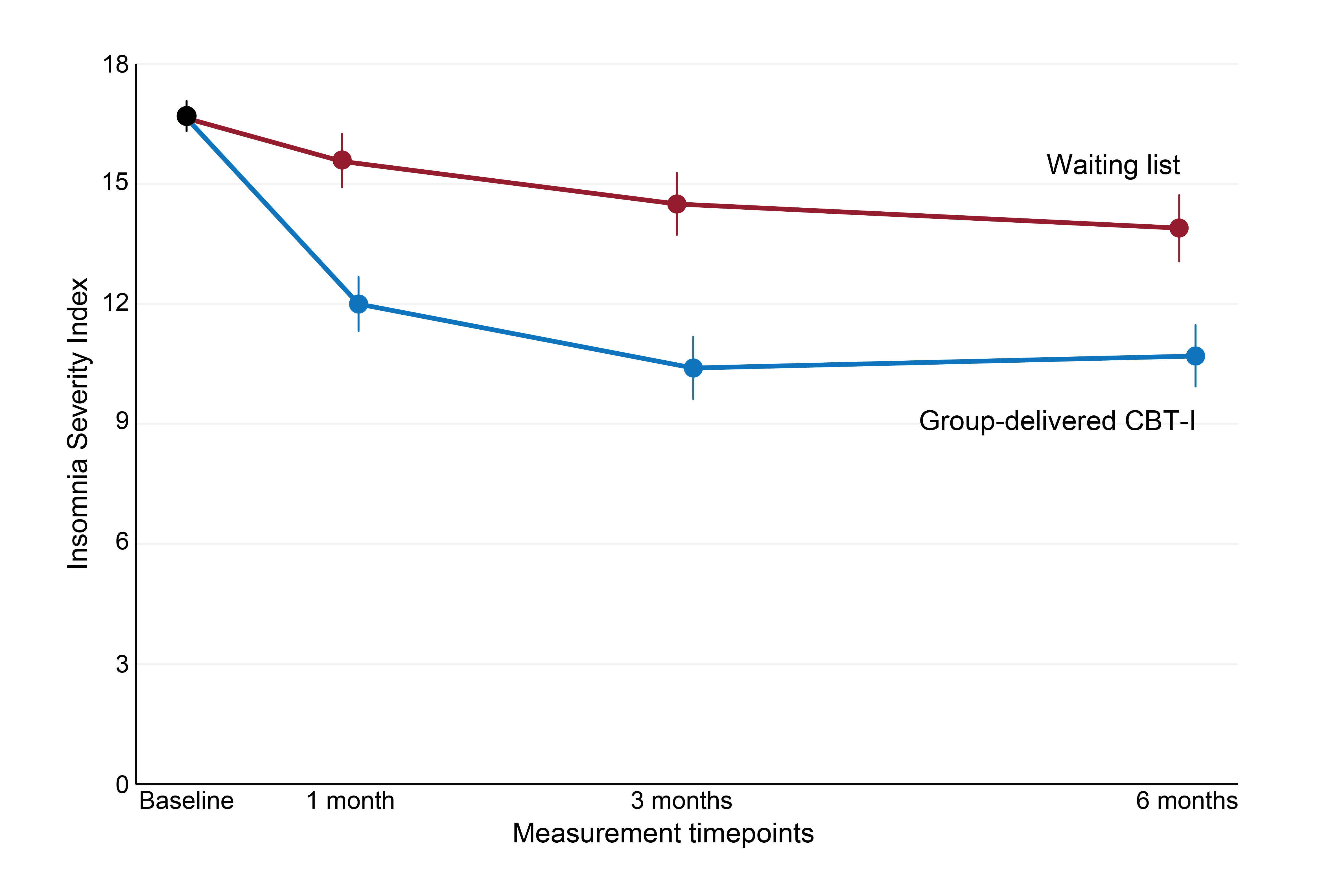
The main outcome of the study was the difference between group-delivered CBT-I and waiting list in the severity of insomnia, measured with the ISI. At 3 months post-intervention, the estimated average difference in the degree of insomnia between the groups was 3.4 ISI points – group-delivered CBT-I participants had a score of 10.7 points, while the ISI score for the waiting list was 14.1 points. This shows that participants who received group-delivered CBT-I had less insomnia at 3 months post-intervention. The effect was maintained at 6 months post-intervention, indicating a good long-term effect of the course (see Figure 2). Based on these results, we can conclude that participation in group-delivered CBT-I leads to less insomnia.
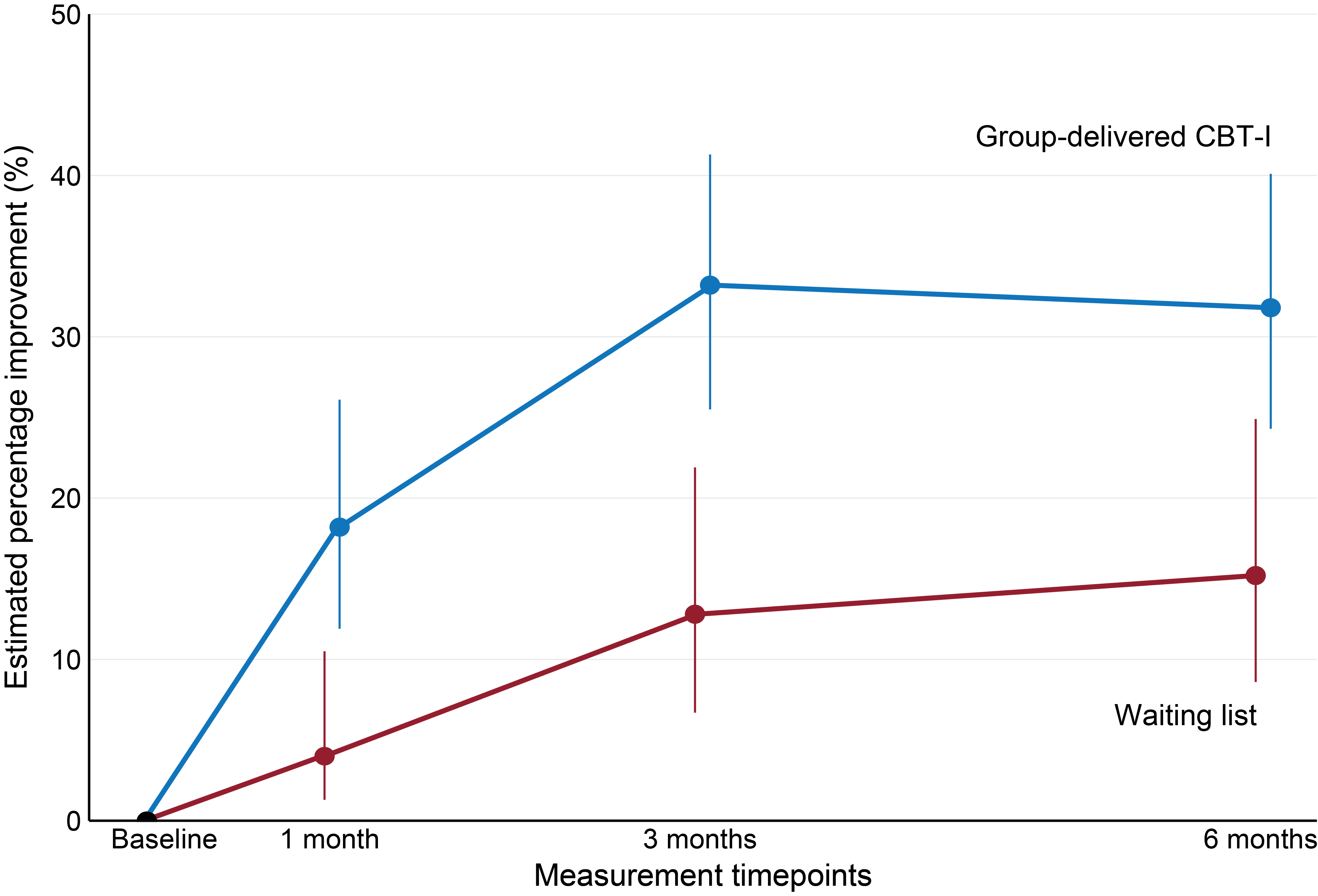
Another important measure in the study was clinically relevant improvement in insomnia, which provides a concrete assessment of the practical effect of group-delivered CBT-I. Clinically relevant improvement in insomnia was defined as a difference of at least 8 points on the ISI scale from baseline to 3 months post-intervention. At 3 months post-intervention, 34% of the group-delivered CBT-I participants had achieved clinically relevant improvement in insomnia, while only 13% of the participants on the waiting list had achieved a similar improvement. This difference was maintained at 6 months post-intervention, indicating a good long-term effect of the course (see Figure 3). Thus, more participants who received group-delivered CBT-I had achieved a clinically relevant improvement in insomnia than the waiting list participants.
Summary of results
This randomized controlled trial is the first to demonstrate the effectiveness of the group-delivered CBT-I course Sleep well provided in Norwegian primary health care. Group-delivered CBT-I reduced the degree of insomnia, and the effect persisted for at least 6 months. The group-delivered CBT-I course Sleep well is therefore suitable as a low-threshold treatment for adults with insomnia in Norway.
Read more
For more details and results showing the effectiveness of group-delivered CBT-I on fatigue, psychological distress, health-related quality of life and self-reported sleep, read our peer-reviewed scientific article published in Sleep Medicine:
Trial resources
- RCT results: Hrozanova, M., Skarpsno, E. S., Follestad, T., Kallestad, H., Pallesen, S., Nordstoga, A. L., ... & Meisingset, I. (2025). Effectiveness of group-delivered cognitive behavioural therapy for insomnia in primary care: a pragmatic, multicentre randomised controlled trial. Sleep Medicine, 106495.
- Study protocol: Hrozanova, M., Meisingset, I., Kallestad, H., Pallesen, S., Nordstoga, A. L., & Skarpsno, E. S. (2023). Group-delivered cognitive behavioural therapy versus waiting list in the treatment of insomnia in primary care: study protocol for a pragmatic, multicentre randomized controlled trial. BMC Primary Care, 24(1), 1-10.
- Trial registration: https://doi.org/10.1186/ISRCTN16185698
Research group
- Nina Elise Møllerløkken, Friskliv og Mestring Trondheim kommune
- Astrid Sletteng Rønning, Friskliv og Mestring Trondheim kommune
Affiliated researchers
- Håvard Kallestad, researcher, NTNU
- Frode Moen, associate professor, NTNU
- Anne Lovise Nordstoga, associate professor, NTNU
- Ståle Pallesen, professor, UiB
