Products and services - Viral Vectore Core Facility - Kavli Institute for Systems Neuroscience
Products and services
Custom viruses
Recombinant viruses can be custom synthesized on a fee-for service basis, when the customer provides transgene construct for virus production. Vector core can assist in research by designing novel constructs and subsequent production of the viruses, please consult the core facility for the details.
For labs outside NTNU: Visit the Viral Vector Core webshop
The webshop is currently not available for labs within NTNU. Please contact us for prices and payment details.
We currently offer the following products
ADENO-ASSOCIATED VIRUSES (AAVs)
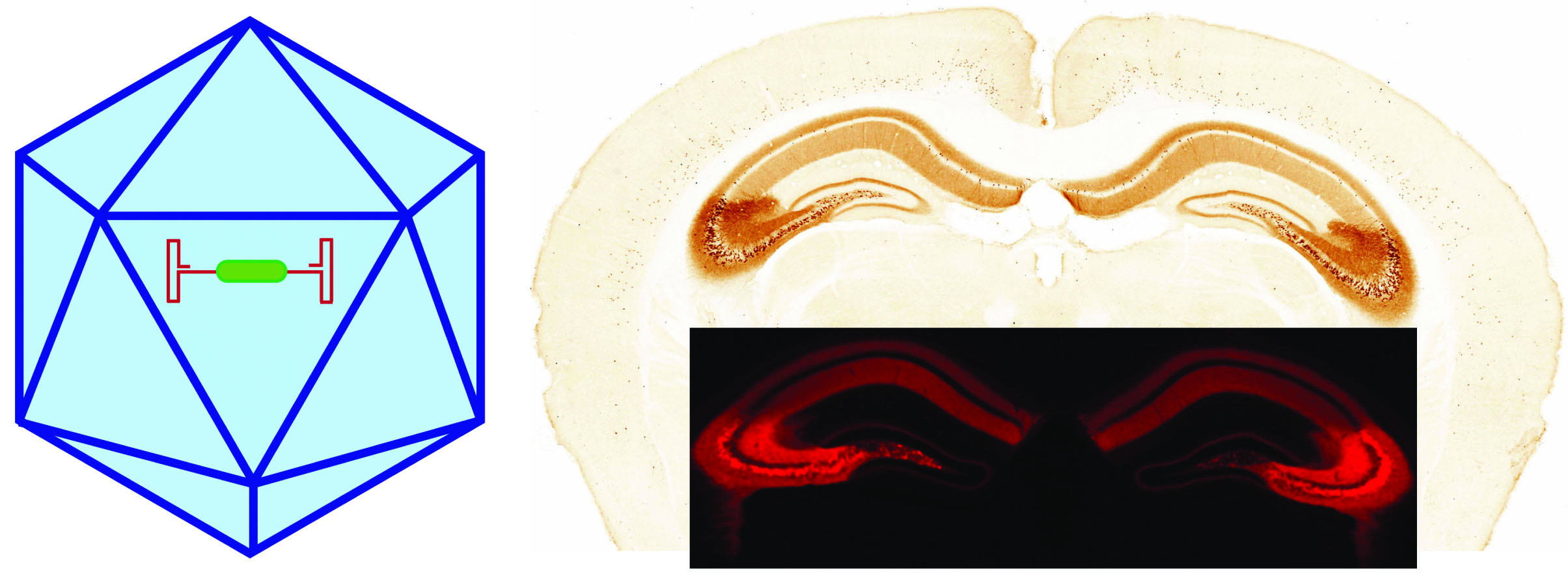
Recombinant AAV is a replication defective, non-enveloped, non-pathogenic ssDNA virus- a very popular viral tool in research and nowadays in clinics. They rarely integrate into the host cell genome and remain primarily episomal.
Recombinant AAV-mediated transgene expression can persist for several years. There is transgene size limitation, it cannot accommodate large DNA sequences (can package a small payload up to 4.5 kb). Efficient for conditional expression of transgenes in combination with transgenics lines. Different serotypes with different cell-specific tropism can be synthesized.
RETROVIRUS (MOLONEY MURINE LEUKEMIA VIRUS AND LENTIVIRUS)
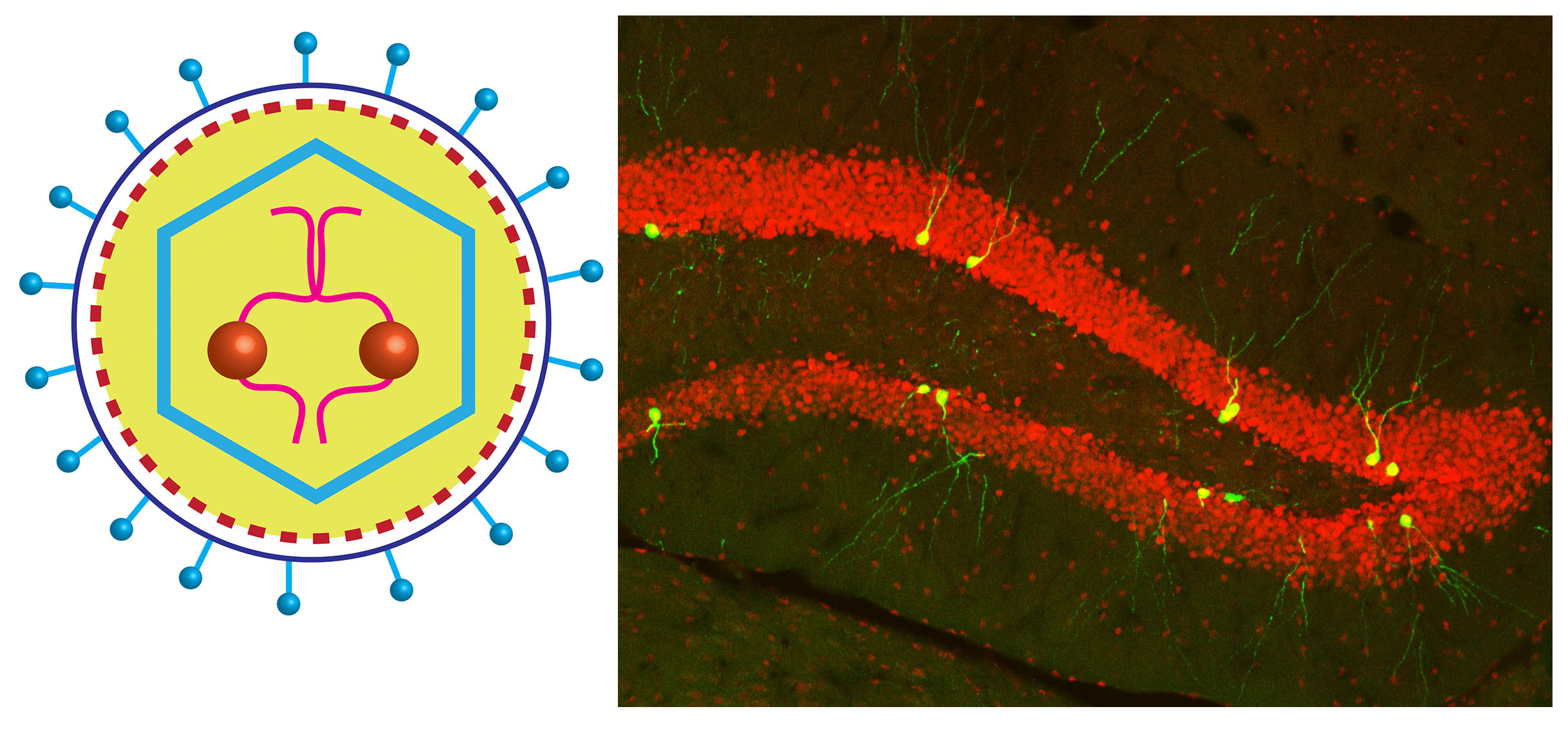
A retrovirus is an enveloped, ssRNA virus. It is mainly used for stable, long-term gene expression because of its ability to stably integrate the viral genome into a host cell’s genome. The expression level is typically low.
The two types of retroviruses commonly used as viral vectors are the gamma-retroviruses (Moloney murine leukemia virus or MoMLV) and lentiviruses. The distinction between these two types of retroviruses is their ability to enter a host cell’s nucleus and subsequent integration of the viral DNA. Gamma-retroviruses (MoMLV) are used to target dividing cells and lentiviruses to target non-dividing cells.
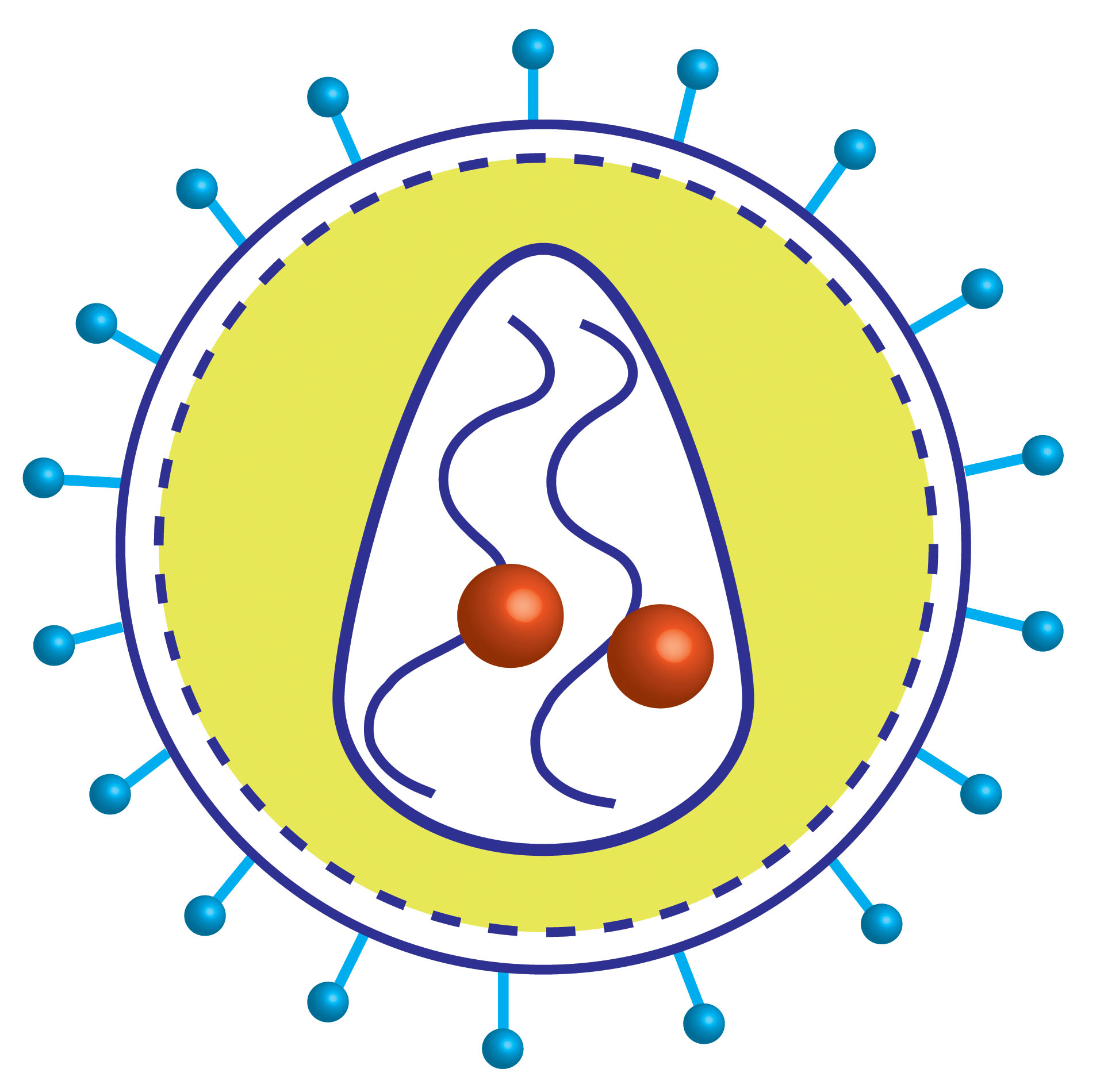
PSEUDOTYPED G-DELETED (∆G) RABIES VIRUSES

Rabies virus is an enveloped, negative sRNA virus. Genetically modified rabies glycoprotein (G)-deleted rabies virus, trans-complemented with rabies-G (provided by transgenics or a helper virus) is used for transsynaptic input tracing.
EnvA-pseudotyped recombinant rabies cannot infect mammalian cells but it will infect only the cells that express exogenous receptor TVA on it surface (starter cells). Subsequent application of EnvA pseudotyped glycoprotein (G)-deleted rabies virus will infect the TVA+ cells and due to the presence of trans complemented rabies-G, the infectious rabies virus are formed in starter cells which will result in monosynaptic spreading of G-deleted rabies viruses to directly connected, presynaptic neurons.
MAXI AND MEGAPREPS OF ENDOTOXIN FREE PLASMIDS
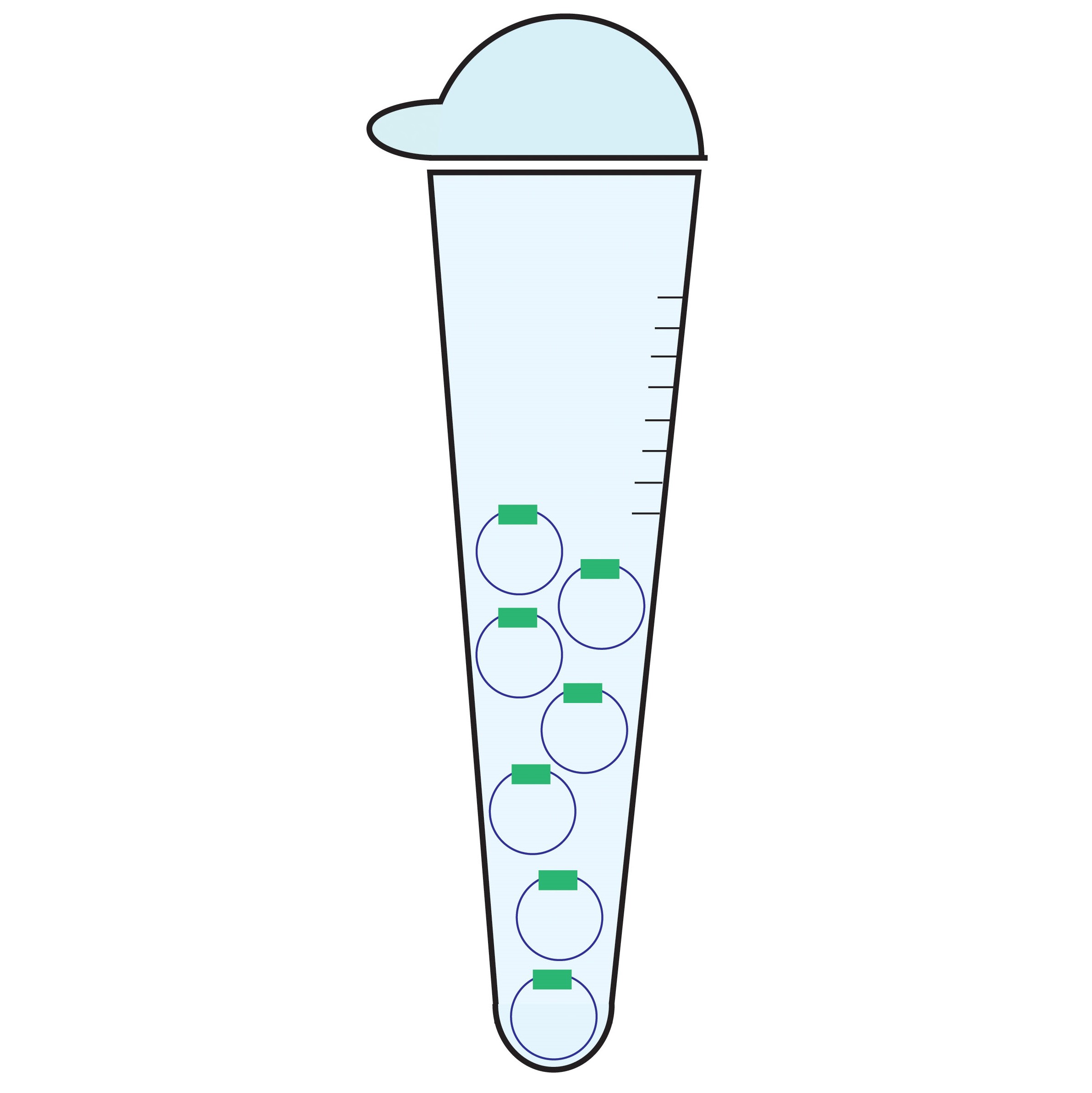
Plasmids are circular, extrachromosomal DNA that can replicate independently in bacteria. They are essential for molecular cloning and subsequent expression of genes of interest in mammalian cells. Endotoxin-free plasmids enables successful transfection and expression of the genes in sensitive cell lines or tissue slices. We offer high-quality transfection-grade plasmid preparations for your research, please contact us for details.
Price list
|
Virus Type |
Internal price* (NOK) |
External price* (NOK) |
|---|---|---|
|
12750 |
13750 |
|
|
12750 |
13750 |
|
|
12750 |
13750 |
|
|
6650 |
7150 |
*Production cost may reduce when more custom viruses are ordered together. Please contact us for details.
Contact the core facility
For inquiries, please contact Core Manager
Dr. Rajeevkumar Nair Raveendran,
rajeevkumar.r.nair@ntnu.no
Visiting address
Kavli Institute for Systems Neuroscience
Olav Kyrres gate 9
7030 Trondheim
Publishing results and co-authorship
Publishing results and co-authorship
Acknowledgement
To ensure our existence, we need to show that scientists are using Viral Vector Core, and that our services are reflected in publications. Viral Vector Core should be acknowledged in publications where tools or data from tools acquired at the Viral Vector Core facility is used. Please use the following sentence to credit Viral Vector Core:
"The xxx virus was obtained from the Viral Vector Core facility of the Kavli Institute for Systems Neuroscience, NTNU"
In addition, the Viral Vector Core facility must be notified when such a manuscript is accepted for publication, so that we can register it in our archive.
Co-authorship
If highly qualified technical personnel or scientific personnel from Viral Vector Core facility contribute with their scientific knowledge to solve the scientific questions, they should be co-authors when the contribution is sin accordance with the "ICMJE Uniform Requirements for Manuscripts Submitted to Biomedical Journals concerning Authorship and Contributorship".
Use of instruments and assisted use of instruments in well known methods and performed by technical staff, is not enough to require co-authorship.
List of publications
List of publications
List of publications
(Applied viral vectors produced at the Kavli Institute for Systems Neuroscience)
Conference presentations
