Ugelstad Laboratory - Colloid and Polymer Chemistry
Ugelstad Laboratory - Colloid and Polymer Chemistry

Ugelstad Laboratory is the group for interfacial, colloid and polymer chemistry at the Department of Chemical Engineering. The laboratory was established in 2002 in memory of late Professor John Ugelstad (known for discovering a process to manufacture monodisperse beads in the µm size range) by Professor Johan Sjöblom. Currently the group is headed by Professor Gisle Øye and brings together leading expertise in computational analysis, state of the art colloidal syntheses of nanoparticles and advanced characterization of interfacial and colloidal systems.
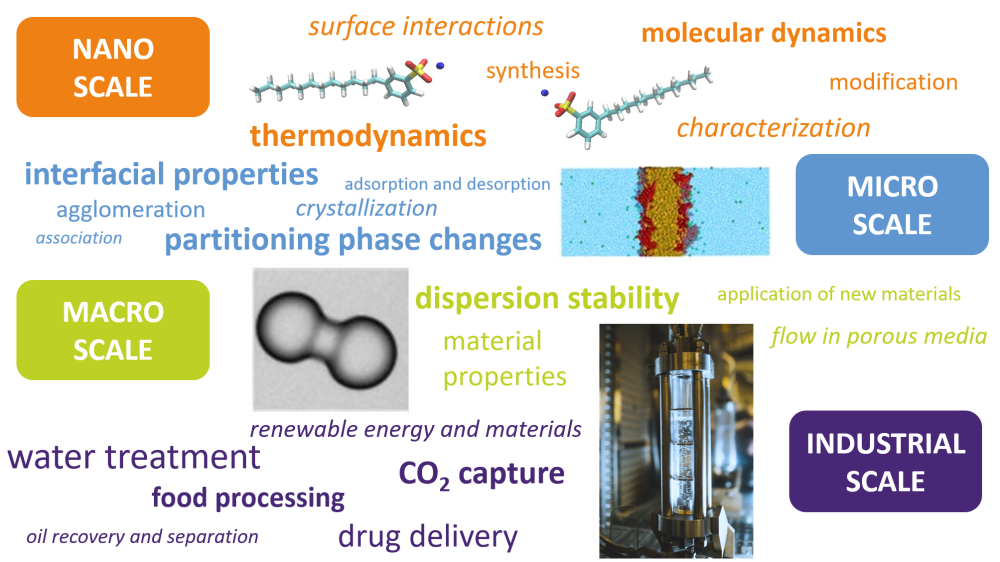
Interfacial and colloidal chemistry provides understanding of natural processes like formation of clouds, rain and lipid membranes; all prerequisites for life. In everyday life we encounter colloidal systems in food, cosmetics, cleaning products, pharmaceutical products and coatings, while industrial applications include transport and processing of oil and gas, wastewater treatment, mineral recovery and paper production. Interfacial phenomena are essential for the quality, efficiency and sustainability of products or processes in all these examples.
The research at Ugelstad Laboratory spans from experimental or simulation studies of dissolved molecules and assemblies of molecules at interfaces, via studies of how the resulting interfacial films influence the stability of colloidal dispersions, ending up with an understanding of how the molecular and interfacial phenomena govern large scale processes such as oil-water separation, water treatment, enhanced oil recovery, food processing, biomedicine and CO2 capture (see illustration).
The group collaborates closely with Norwegian and international industry, research institutes (IFE, RISE-PFI, SINTEF) and academic groups throughout the world. We continuously invest in new instrumentation, develop novel experimental methodologies and maintain high HSE-standards in our laboratory facilities. In this way, Ugelstad Laboratory offers a unique and ambitious research environment for students and young scientists, educating highly qualified Master and PhD candidates for industrial and academic positions in the cross section between interfacial and colloid chemistry and chemical engineering.
Interfacial Engineering
An interface is the boundary between two phases where the properties are different from the adjacent phases. A colloidal dispersion is a multiphase system where particles, drops or bubbles, with sizes between 1 nm and 1 µm, are dispersed in a continuous matrix. Such suspensions, emulsions or foams can have enormous interfacial areas, which means that phenomena occurring at the interfaces are essential for the behaviour of these systems. Examples are interfacial tension, interfacial rheology, interfacial adsorption and desorption and interfacial forces, which are related to the stability of the dispersions. The stability must be promoted to prolong shelf-life and avoid alterations of texture and taste during the design of food products, for instance. In water treatment processes, on the other hand, the dispersions must be destabilised to obtain phase separation as efficiently as possible. The research at Ugelstad Laboratory is devoted to understanding how the efficiency of industrial processes and technical systems are linked to interfacial properties at the molecular scale.
Complex interfaces in stability and transport of dispersions
Industrial fluids are complex in composition, resulting in complex interfaces. The interfacial tension and interfacial rheology are important factors during microscopic processes like emulsion formation and coalescence of droplets, which again govern macroscopic processes like separation of gas, oil and water during petroleum production. At Ugelstad laboratory we are in the international research front when it comes to interfacial investigations and understanding the relationships between interfacial properties and separation efficiency of petroleum emulsions. Utilising renewable materials like lignosulfonates as stabilisers for particles and droplets is another example of our work on complex interfaces.
Complex interfaces will also influence transport of dispersions. When petroleum crude oil is produced, for example, it must be transported by pipeline from the wells to the processing plants or point of sales. During this transport various types of solids (asphaltenes, waxes, gas hydrates, naphthenates, scales) can form and lead to plugging of the pipeline and hinder transport. Specific methods must be implemented to prevent or remedy their formation (this is often called flow assurance). At Ugelstad Laboratory we determine the mechanisms of formation of these solids and develop new analysis and inhibition methods in collaboration with key oil companies and chemical vendors.
Complex interfaces in multiphase flow in porous media
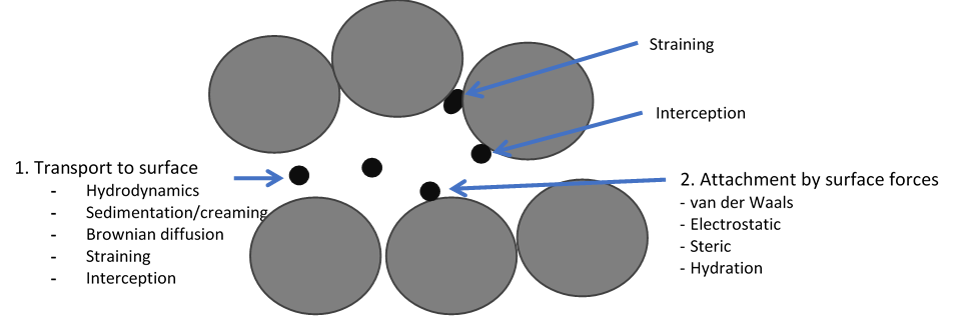
Displacement of one fluid by another immiscible fluid in porous structures is essential for processes such as enhanced oil recovery, contamination of ground water and geological CO2 sequestration. Transport and retention of drops, solids and bubbles in porous structures are determining the efficiency of processes such as re-injection of produced water into reservoirs during petroleum production (see illustration), membrane emulsification and membrane filtration. We develop advanced microfluidic methods for studying these phenomena at Ugelstad Laboratory.
Microfluidics
Microfluid methodology is a supreme way of controlling and visualizing the behaviour of fluids and dispersions in micrometer sized channels and networks. In addition, it can offer a faster and more accurate measurements then traditional measurement methods due to fast heat and mass transfer. Currently, we are developing microfluidic methods as a viable way of studying multiphase systems at timescales, temperatures and pressures relevant for industrial processes. More specifically; investigations of drop-drop and drop-bubble coalescence, phase displacement and dispersions in porous media, which are important phenomena in produced water treatment, gas flotation, enhanced oil recovery and re-injection of produced water, respectively. Furthermore, we use microfluidics in the development of solar cells.
Rheology
Rheology is the study of deformation and flow of continuum matter. Complex fluids often exhibit unique rheological behavior which is attributable to the interfacial-intensive material structure. Rheological investigations provide a bridge between material chemistry and large-scale flow behavior/hydrodynamics in industrial systems. Pristine wax-oil gels exhibit “irreversible non-ideal thixotropy”, which is manifested in a deformation-dependent structural state. During restart of gelled oil pipelines, deformation-dependent gel rheology gives rise to a coupled pressure wavefront which combines the properties of a diffusive viscous compression wavefront and a rheological degradation wavefront. The rheology of wax-oil gels modified by inhibitors and heavy polar components is a current topic of research at Ugelstad Laboratory. In addition, drilling fluid rheology and resultant cleaning performance is a research topic at Ugelstad Laboratory.
Lab equipment
The group is running state-of-the art laboratories for interfacial and colloidal characterization of dispersed systems such as emulsions, suspensions, nanoparticles and foam. More information on the specific instruments used for the various types of analysis and characterization can be found on BookItLab.
Microfluidics
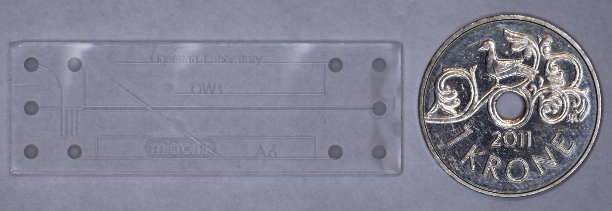
Microfluidics deals with observation and control of fluids in small channels. It is a technique with a wide range of applications, such as performing reactions on a microscale, probing emulsion stability, measuring interfacial properties or following flow in porous media. Here is an example of a microfluidic chip, compared to a Norwegian 1-krone coin.
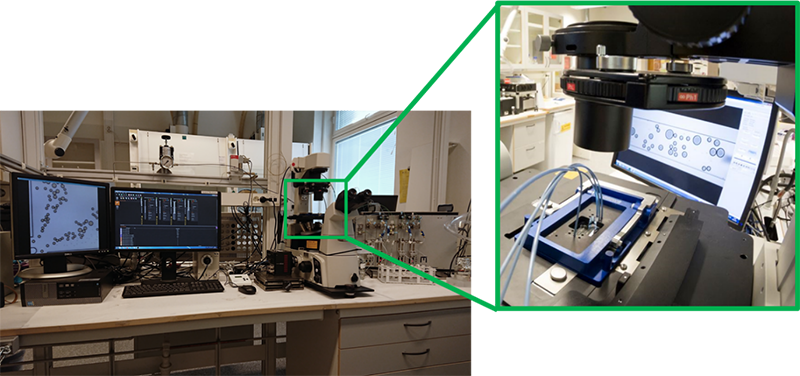
At Ugelstad Laboratory, we utilize microfluidics to study different kinds of phenomena such as flow properties and stability of droplets and bubbles, oil displacement from network-based chips, online spectroscopic analysis of fluids and retention of droplets in porous media (two videos or gifs here). To do this, we use microfluidic platforms. These multi-instrument devices are typically composed of a microscope and a high-speed (up to 500k frames per second) or high-resolution camera for observation; and pumps, valves, sensors, spectrophotometers and other auxiliary equipment for controlling the flow, composition, temperature, pressure, and the state of fluids injected into the microfluidic chips.
Tensiometry and adsorption techniques
Dynamic and equilibrium interfacial tension measurements describes adsorption onto interfaces and can be performed by various methods. The choice of method often depends on the sample and the timescale which is of interest. At Ugelstad Laboratory we have four different ways of measuring interfacial tension.
Ring tensiometer allows to perform measurements of surface (air-liquid) or interfacial tension (liquid-liquid). It relies on measuring the force required to pull the surface/interface to a maximum point (Samuel’s animation with the ring and force graph). Two titrators that enable automatic measurements of the critical micelle concentration can also be attached to avoid a number of time-consuming measurements.
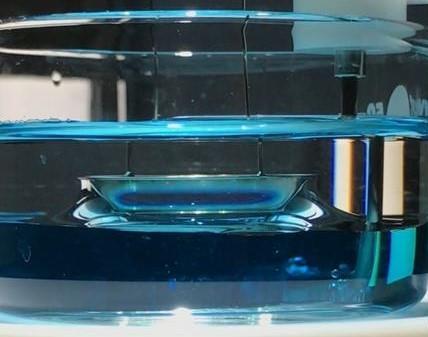
Spinning drop tensiometer relies on image analysis ofa drop of a lighter fluid injected in a revolving capillary, filled with a heavier fluid. The drop will be elongated with increasing speed of revolution as the centrifugal force will increasingly oppose the drive of the interfacial tension towards a minimum interfacial area. The technique is useful for measuring low and ultralow interfacial tensions. (video of a drop being contracted and elongated).
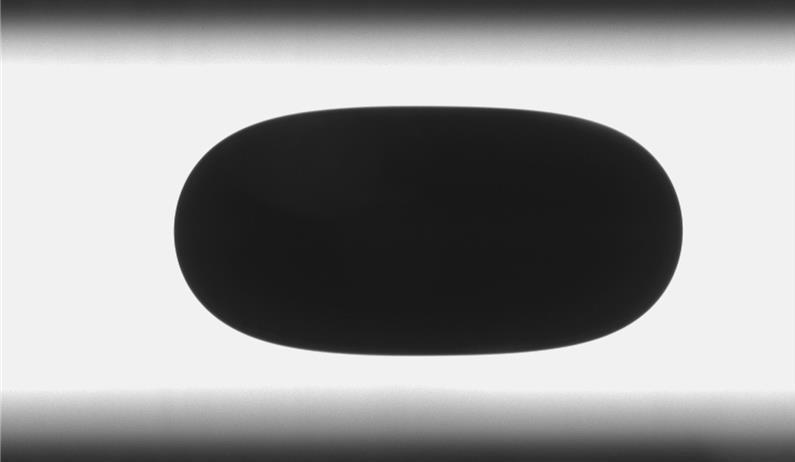
Another drop method available in our group is pendant drop tensiometer, particularly suitable for time-dependent measurements of adsorption of surfactants at liquid/air or liquid/liquid interfaces. Images of a drop created from a capillary are continuously recorded and resolved for interfacial tension. The rheological properties (elasticity and viscosity) of the interface can also be determined by subjecting the drop to periodic oscillation of the volume. The setup can also be used to measure contact angles on a flat substrate.
Maximum bubble pressure tensiometer allows to access values of surface tension on a millisecond scale. Bubbles generated from a capillary are immersed into a solution and the maximum pressure during the bubble formation is detected and used to calculate the surface tension. With this information, surface characteristics and diffusion kinetics can be calculated.
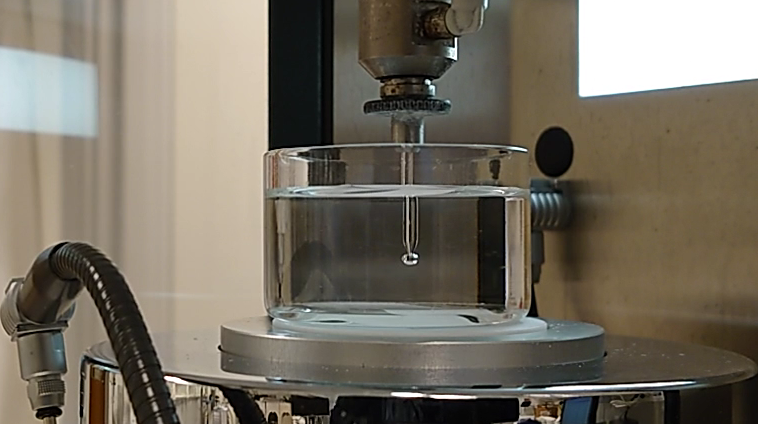
Quartz Crystal Microbalance enables direct studies of adsorption at solid surfaces. The measurements are based on changes in vibration frequency of a quartz crystal sensor in response to adsorption on the sensor surface. The technique can be used to follow molecular events in real-time, measure mass and thickness of molecular layers and analyze mechanical properties of adsorbed layers.
Particle and dispersion characterization
Dispersion characterization and stability plays a central part in many of the activities in our research group. Therefore, the laboratory is equipped with a number of instruments that allow to study dispersed systems in various ways.
Several of our instruments rely on light scattering phenomena, i.e. reflection of light from the surface of dispersed particles and its subsequent detection. This can provide different kind of information, such as turbidity (transmission of light through the sample) and particle size (obtained from the angle of scattered light or diffusion of colloids).
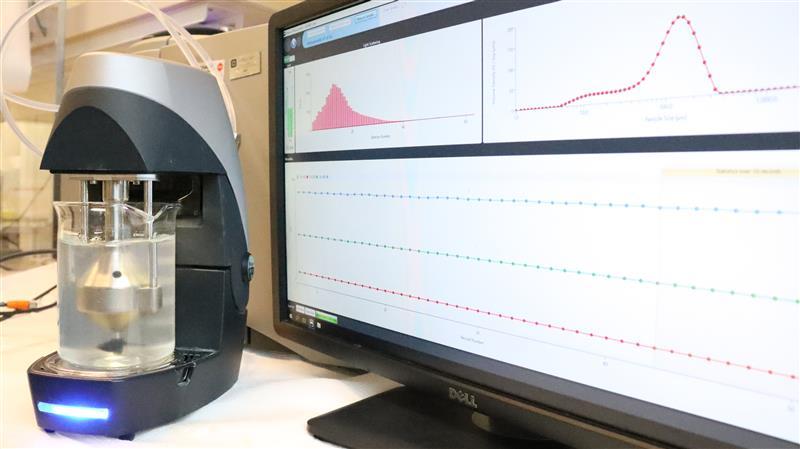
Furthermore, many of these methods allow for resolving changes of the properties over time, which gives access to information about the sedimentation or creaming, as well as coalescence, flocculation, and drop/bubble breakage.
While most aqueous samples can be probed with the above-mentioned techniques, characterization of opaque samples is a tougher challenge. Still, both size distributions and the stability of these kinds of dispersions can be probed with nuclear magnetic resonance (NMR) spectroscopes, available at our facilities.
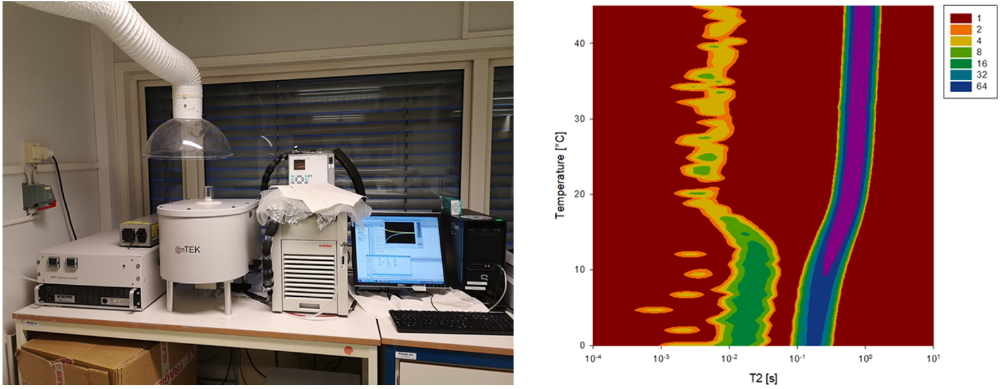
Other type of characterization techniques available at the lab include measurements of electrophoretic mobility (zeta potential), rheometry, and microscopic analysis.
Spectroscopical and optical techniques
For spectroscopic characterization of liquids and powders, UV-vis spectroscopy (190 - 1100 nm), FT-IR with ATR cell (7500 – 370 cm-1), Near-Infrared spectroscopy (12800 – 4000 cm-1) and Raman spectroscopy (325, 633 and 785 nm, 10, 15 and 50x objectives, fiber optic head for liquids) are available.
Steady-state absorption and emission spectroscopy, as well as fluorescence lifetime measurements by time-correlated single photon counting can be obtained on our Fluorescence Spectrophotometer for liquid samples.
Chromatographic methods
High performance liquid chromatography: . A preparative HPLC setup has been specially designed to quantify the saturate, aromatic, resin, and asphaltene fractions (SARA) of crude oils. This fractionation is generally used as one of the first techniques to characterize the composition of crude oil. After removing the asphaltenes from crude oil by precipitation in a solvent, the saturates, aromatics, and resins are fractionated with the preparative HPLC using a silica and an amino column with a dual UV and refractive index (RI) detection. In addition to the preparative HPLC there is an analytical HPLC with a size exclusion column (SEC) for analysis of unknown samples based on molecular size.
A GC-GC-MS (two columns) for chromatographic separation and simultaneous analysis by FID and MS is available.
Thermal analysis

Differential Scanning Calorimeter: The instrument measures heat flows associated with thermal transitions in a material as a function of temperature. Properties that can be determined include glass transitions, melting, crystallization, asphaltene precipitation kinetics, and paraffin wax analysis.
Isothermal Calorimeter is a thermodynamic technique that directly measures the heat released or absorbed during a molecular binding event. It is used to determine thermodynamic parameters such as enthalpy and equilibrium constants and to study interactions and self-association of molecules.
Miscellaneous
Many other basic techniques are available, such as: Karl Fisher Coulometer for determination of small amounts of water in emulsions, Critical Electric Field Emulsion Stability Cell for testing stability of water-in-oil emulsions, Density/Concentration Meter for measuring the density of solutions, Titrando titration device to determine the total concentration of acids (TAN) or bases (TBN) present in a sample by titration, pH Meter for measuring pH in solutions, Rotavapor to separate liquids by applying vacuum and heat, Conductivity Meter to measure electrical conductivity in solutions and a 3D-printer for rapid prototyping.

Ugelstad Laboratory Group Photo 2022

Scientific Staff
 |
Professor Gisle Øye Head of Ugelstad Laboratory Colloid chemistry and interfacial phenomena within Produced Water Managament, Enhanced Oil Recovery and synthesis of nanostructured materials. |
 |
Associate Prof. Nadia Shardt
|
|
|
Professor Kristofer Gunnar Paso Research interests: Polymer technology, Nanocellulose and Rheology. |
 |
Associate Prof. Brian Arthur Grimes Specializes in molecular dynamics simulation, adsorption, and transport phenomena. Applications include crude oil separation/flow assurance, chromatography. |
 |
Adjunct Prof. Kristin Syverud Research manager at RISE PFI, www.rise-pfi.no/, and lead scientist of the focus area Nanocellulose and Carbohydrate Polymer.
|
Researchers
 |
Colloid chemistry with a focus on petroleum emulsion, crude oil chemistry, nanofibril cellulose, naphthenic acids, and naphthenate deposits.
|
Technical Staff
|
|
Senior Engineer Contact for external users of the laboratory facilities, Administration of the local "Leiested", Laboratory engineer with Instrument responsibility (DSC, rheometer, QCM-D, and others)
|
PhD Candidates
 |
Gas flotation for subsea produced water treatment |
 |
Flow Improvers for Waxy Crudes |
 |
A Digital Twin Library for Oil/Water Emulsion Separation and Transport Processes |
 |
Optimized wellbore dynamics – Rheological behavior of drilling fluids and cutting beds |
Education - We coordinate the following courses:
Spring semester
Surface- and Colloid Chemistry (TKP4115)
The course gives an introduction to important principles of surface and colloid chemistry. Coordinator: Professor Gisle Øye
Polymer Chemistry (TKP4130)
The course provides an introduction to polymer chemistry. Coordinator: Associate Professor Kristofer Paso
PhD courses
Doing Science: Methods, Ethics and Dissemination (MN8000)
The course is aimed to train all PhD students in natural sciences and technology students to become eminent scientists, to understand the role of science in society, and to perform and communicate their science efficiently and ethically. Coordinator: Associate Professor Brian Grimes
Surface- and Colloid Chemistry (KP8905)
The course gives an introduction to important principles of surface and colloid chemistry and provies deeper insight into a selected topic. Coordinator: Professor Gisle Øye
Autumn semester
Colloid and Polymer Chemistry, Specialization Course (TKP4525)
The specialization subject consists of elective modules which are to be combined to yield a total of 7.5 study points (stp). The elective modules are:
Industrial colloid chemistry (3.75 stp).
Surfactants and polymers in aqueous solution (3.75 stp).
Modules from other subjects/courses within the department or from other departments/faculties can be selected provided agreement from the coordinator.
Transport Phenomena (Second degree level: TKP4160)
Governing equations for momentary mass and heat transport. Laminar and turbulent flow, laminar and turbulent boundary layer. Brief introduction to rheology and non-Newtonian fluids for biological systems. Steady and un-steady diffusion in dilute and concentrated fluids in different geometries. The Fick and Stefan-Maxwell equations, multicomponent diffusion. Diffusion in porous media. Generalised equations for momentum, mass and heat flow. Laminar and turbulent boundary layers. Mass transfer models. Simultaneous heat and mass transfer and transfer analogies. Introduction to Matlab (Solving ordinary differential and partial differential equations, discretization).
PhD courses
Colloid Chemistry for Process Industry (KP8129)
The course gives an introduction to how surface and colloid chemical phenomena influence industrial processes. Coordinator: Professor Gisle Øye
SFI SUBPRO - Subsea Production and Processing Centre
- Effect of production and EOR chemicals on produced water quality
- Emulsions in porous media, produced water reinjection
- Multiphase Separation and transport model library
Petromaks II
- ELCO - New strategy for separation of complex water-in-crude oil emulsions – from bench to large scale separation
- Green EOR - Green high-performance systems for Enhanced Oil Recovery
- Optimized hydraulic behaviour in well construction
NANO2021
- NanoVisc - Development of high-performance viscosifiers and texture ingredients for applications based on cellulose nanofibrils
- Development of ionic liquid super capacitors
Other
- Development of novel microfluidic methods for investigating mobilisation and displacement mechanisms in EOR processes (VISTA)
- Ligno2G: 2nd generation performance chemicals from lignin (BIA)
- CO2 capture in confined geometries (CLIMIT)
- Microfluidic Augmented Nanostructured Solar Cells for Low-Cost Sustainable Energy Alternatives (NV faculty)
- Understanding the growth and properties of hybrid nanomaterials for biomedical applications (NV faculty)
- Wax crystallisation with inhibitors
Coalescence and contact time measurements with microfluidics
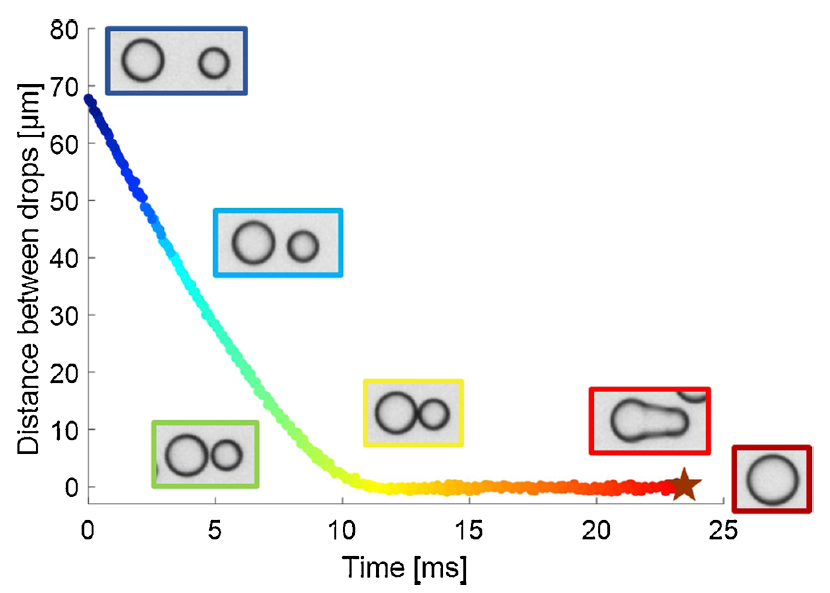
Marcin Dudek et al. have developed a microfluidic method that allows to measure thousands of coalescence and contact times between flowing droplets in an emulsion. While there are other techniques for measuring coalescence times, most of them require static conditions and struggle with producing statistically significant amount of data. Amongst other things, they showed how the coalescence time changes with the size of droplets or the effect of temperature on coalescence time distributions. https://doi.org/10.1016/j.colsurfa.2019.124265
Microfluidic method for studying oil recovery
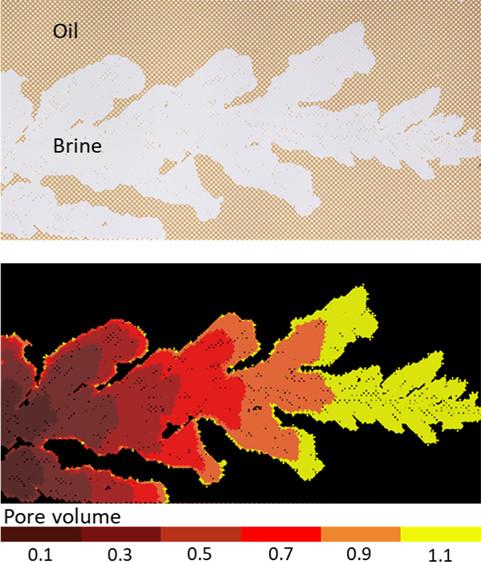
In this paper, Marzieh Saadat et al. demonstrated the potential of microfluidics for studying oil recovery processes. The recovery was observed with a camera, allowing to visually follow the progress of the flood and calculate the residual oil at different stages. Several aspects, such as flood characteristics, injection rate and aging of the oil in the chip were investigated and compared to conventional, time-consuming core floods. https://doi.org/10.1021/acsomega.0c02005
Dye-sensitized solar cells augmented with microfluidic flow

Gregory Rutkowski and Brian Grimes fabricated dye-sensitized solar cells with microfluidic channels that enabled to circulate electrolyte, which significantly improved the photocurrent generation. What is more, this allowed to regenerate the degraded photoactive dye layer and recover the initial performance of the cells. The accompanying picture was selected for the issue cover image of ACS Applied Energy Materials. https://doi.org/10.1021/acsaem.9b00952
Coalescence of water droplets in electric field
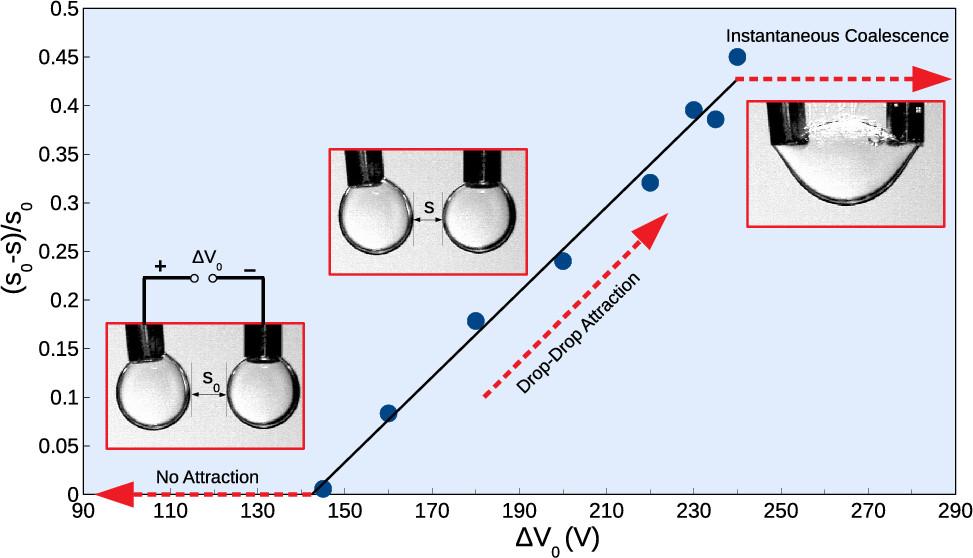
The work of Sameer Mhatre et al. presented work on electrocoalescence of water droplets in the presence and absence of asphaltene molecules, demulsifiers, and under different electric potentials. Each system was described with specific critical voltage of coalescence, depending on the composition of the phases, as well as other conditions, for example droplet aging. Both the method and the result are an important step to understanding the complexities of electrocoalescence processes. https://doi.org/10.1021/acs.langmuir.9b03554
Lignosulfonates as “green” emulsion stabilizers
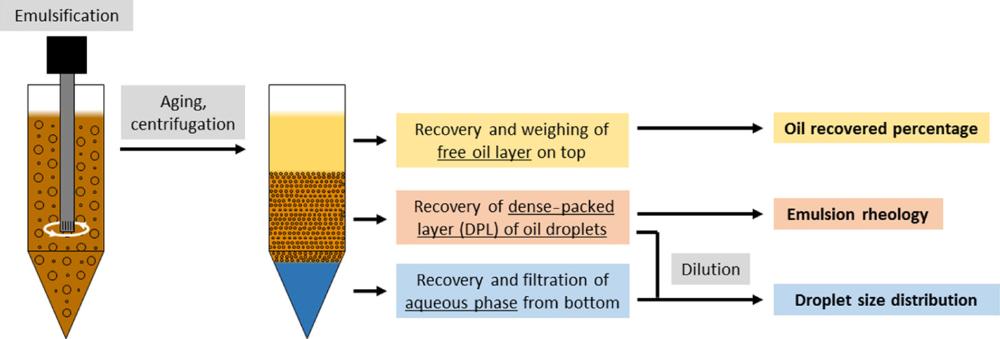
Jost Ruwoldt et al. investigated the properties and stability of emulsions with renewable and biodegradable lignosulfonates used as stability improvers. Several types of additives were tested, representing various molecular weight, structure and hydrophobicity of the lignosulfonates. Overall, the results showed large potential for their application in emulsion stabilization processes. https://doi.org/10.1021/acsomega.0c00616
Replacing fat with cellulose nanofibrils in mayonnaise
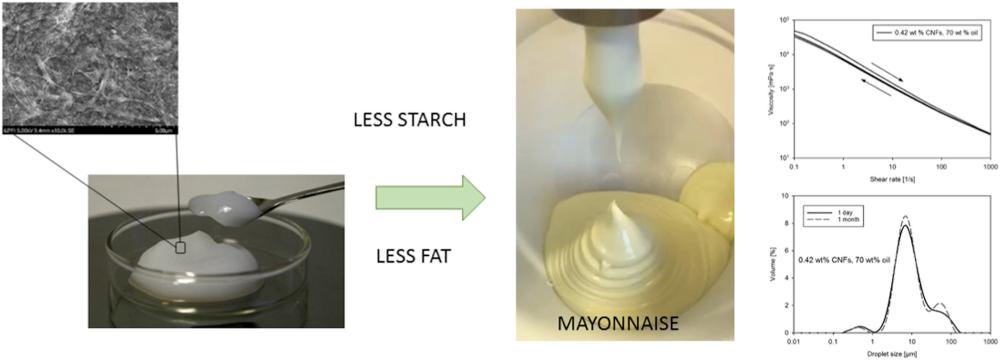
Ragnhild Aaen et al. studied the effect of adding cellulose nanofibrils as an attempt to reduce the fat content in mayonnaise. The test samples, manufactured at Mills food plant, were compared with standard mayonnaise with regard to viscosity and change of droplet size over up to one month. The authors concluded that the properties of the modified mayonnaise were very close to the standard samples, which indicated the possibility of using cellulose nanofibrils in this product. https://doi.org/10.1016/j.foodhyd.2020.106084
Gas flotation for treatment of petroleum produced water
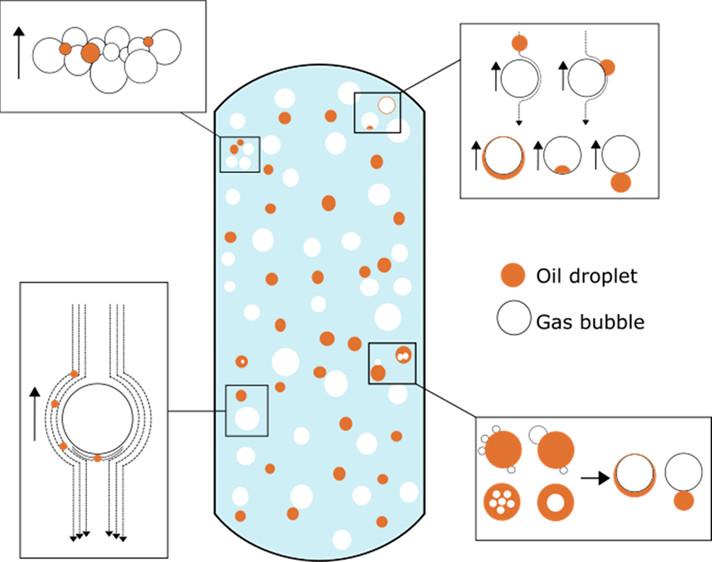
A review paper by Martina Piccioli et al. in detailed described the process of gas flotation, which is one of the common ways to remove small oil droplets from produced water before discharging it to the sea or re-injecting to a reservoir. The manuscript combined the macro- and microscopic aspects of this process, showing the underlying phenomena behind removal of oil drops by gas bubbles. The authors also discussed the challenges and perspectives of this technique for subsea produced water treatment. https://doi.org/10.1021/acs.energyfuels.0c03262
Academic Staff
-
Brian Arthur Grimes Associate Professor
+47-73590338 brian.a.grimes@ntnu.no Department of Chemical Engineering -
Kristofer Gunnar Paso Professor
+47-73593147 kristofer.g.paso@ntnu.no Department of Chemical Engineering -
Nadia Shardt Associate Professor
+47-73412193 nadia.shardt@ntnu.no Department of Chemical Engineering -
Gisle Øye Professor
+4797567011 gisle.oye@chemeng.ntnu.no Department of Chemical Engineering


